Corrosion protection methods aim to minimize or eliminate corrosion’s harmful effects by implementing various measures to protect materials against corrosive agents. The selection of a particular corrosion protection method depends on the type of material, the environment in which it will be used and the potential sources of corrosion.
What is Corrosion?
Corrosion is a dangerous and extremely costly problem. Because of it, buildings and bridges can collapse, oil pipelines break, chemical plants leak, and bathrooms flood. Corroded electrical contacts can cause fires and other problems, corroded medical implants may lead to blood poisoning, and air pollution has caused corrosion damage to works of art around the world. Corrosion threatens the safe disposal of radioactive waste that must be stored in containers for tens of thousands of years.
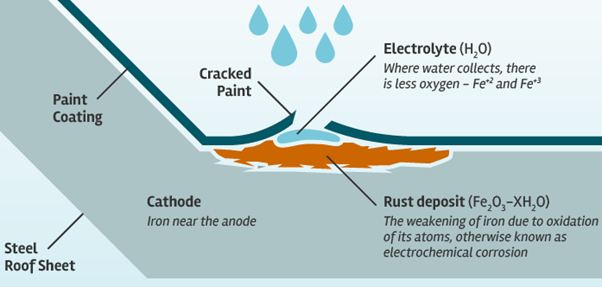
The most common kinds of corrosion result from electrochemical reactions. General corrosion occurs when most or all of the atoms on the same metal surface are oxidized, damaging the entire surface. Most metals are easily oxidized: they tend to lose electrons to oxygen (and other substances) in the air or in water. As oxygen is reduced (gains electrons), it forms an oxide with the metal.
When reduction and oxidation take place on different kinds of metal in contact with one another, the process is called galvanic corrosion. In electrolytic corrosion, which occurs most commonly in electronic equipment, water or other moisture becomes trapped between two electrical contacts that have an electrical voltage applied between them. The result is an unintended electrolytic cell.
Take a metal structure such as the Statue of Unity. It looks strong and permanent. Like nearly all metal objects, however, it can become unstable as it reacts with substances in its environment and deteriorates. Sometimes this corrosion is harmless or even beneficial: the greenish patina that covers the statue’s copper skin protected the metal beneath from weather damage. Inside the statue, however, corrosion caused serious harm over the years.
Natural Protection
Some metals acquire a natural passivity, or resistance to corrosion. This occurs when the metal reacts with, or corrodes in, the oxygen in air. The result is a thin oxide film that blocks the metal’s tendency to undergo further reaction. The patina that forms on copper and the weathering of certain sculpture materials are examples of this. The protection fails if the thin film is damaged or destroyed by structural stress — on a bridge, for example — or by scraping or scratching. In such cases the material may re-passivate, but if that is not possible, only parts of the object corrode. Then the damage is often worse because it is concentrated at these sites.
Harmful corrosion can be prevented in numerous ways. Electrical currents can produce passive films on metals that do not normally have them. Some metals are more stable in particular environments than others, and scientists have invented alloys such as stainless steel to improve performance under particular conditions. Some metals can be treated with lasers to give them a non-crystalline structure, which resists corrosion. In galvanization, iron or steel is coated with the more active zinc; this forms a galvanic cell where the zinc corrodes rather than the iron. Other metals are protected by electroplating with an inert or passivating metal. Non-metallic coatings — plastics, paints, and oils — can also prevent corrosion.
Types of Corrosion
To understand how to prevent corrosion, it is important to know the difference between the different types of corrosion. Knowing which type of corrosion, you are dealing with will help to best determine how it can be prevented.
1. Uniform Corrosion: The most common type of corrosion, uniform corrosion is identified by its reddish color distributed evenly throughout the exposed part of the fastener.
Prevention: To prevent uniform corrosion it is important to allow for water run-off and good ventilation when designing. Also, prevent continuous condensation by keeping surfaces clean and protect fasteners from the beginning with plating’s or coatings.
2. Crevice Corrosion: Crevice corrosion occurs because small gaps and recesses tend to draw in moisture and do not have good ventilation. When crevice corrosion occurs, the risk for crevice corrosion multiplies with the number of joint faces.
Prevention: Crevice corrosion can be avoided by minimizing the use of washers and making joint interfaces as smooth as possible. Another strategy which helps is adding a gasket or sealer between the clamped materials.
3. Galvanic Corrosion: Galvanic corrosion is a result of two dissimilar metals in the presence of moisture.
Prevention: This type of corrosion can be prevented by using fastener materials or protective finishes that are either as noble or more noble than the joint. You can also use plastic washers where clamp load is not critical or avoid using stainless steel or copper parts with zinc plated fasteners.
4. Pitting Corrosion: Pitting corrosion occurs on a metal surface that is coated by a very noble finish like nickel or chromium. The exposed area becomes less noble than the much larger area around it that is less affected by environmental factors. This creates a current density allowing for galvanic corrosion in the pits.
Prevention: To prevent Pitting Corrosion always keep surfaces clean and smooth. Avoid solid or liquid residues, especially chlorides and use A4 or 316 stainless steel when there can be chlorides present. Also, work with your nickel plater to use subsequent treatments that can fill pores.
5. Intergranular Corrosion: Austenitic stainless steels can develop intergranular corrosions when they are heated to a high temperature for hot forming or welding.
Prevention: For intergranular corrosion prevention, use stainless steel with carbon content below 0.05% and quench parts in water immediately after heating. If using stainless steel containing over 0.05% carbon it can be stabilized by adding titanium, niobium or tantalum.
6. Stress Corrosion Cracking: Stress corrosion cracking may result when corrosion occurs on
fasteners subjected to tension. This type of failure often starts with pitting corrosion.
Prevention: Stress corrosion prevention can start with following the guidelines for pitting corrosion prevention. In addition to those guidelines, periodically inspect safety critical parts, hot dip galvanizing if necessary, to check for corrosion. Always ensure that these safety critical fasteners are accessible for inspection and replacement.
7. Hydrogen Embrittlement: While not a form of corrosion Hydrogen embrittlement in a fastener can be a result of corrosion in a joint. If high strength fasteners are stressed, small surface defects like scratches may turn into a small crack. If hydrogen is present in the steel atoms, they are attracted by the tensile stresses around the tip of the crack and form a “hydrogen atom cloud” there. The hydrogen weakens the microstructure of the metal and the crack may continue to grow until the part fails.
Prevention: To prevent Hydrogen embrittlement, avoid electroplating or the use of acid cleaning for high strength fasteners. If Hydrogen is introduced in large quantities to a high strength fastener the possibility of embrittlement is very prominent.
Above all, make fastener choice a priority during the initial stages of the design processes to avoid chance of corrosion from the start.
Some Common Corrosion Protection Methods Include:
- Protective Coatings. Protective coatings are applied to a material’s surface to create a barrier between the material and the environment, preventing direct contact and reducing the corrosion rate. Examples of protective coatings include paints, lacquers and varnishes.
- Cathodic Protection. This technique involves applying an external electrical current to the metal surface to reduce the corrosion rate. This is achieved by using a sacrificial anode or an impressed current system.
- Corrosion Inhibitors. These chemical compounds are added to the environment to reduce the corrosion rate by interfering with the chemical reactions between the metal and the corrosive agent.
- Material Selection. Choosing the right material for a specific application based on its resistance to corrosion. Materials that are commonly used for their corrosion resistance include stainless steel, titanium and aluminium. Asset managers may also opt to replace parts with a suitable inert material, such as plastic etc.
- Environmental Modifications. Changing the environment by adjusting temperature, humidity or pH levels to reduce the corrosive agent’s concentration.
- Design Modifications. One of the best ways to prevent corrosion is to keep it in mind from a project’s design phase — for example, avoiding cracks and pits where the metal can hold water, or placing a non-metallic washer between two metals far part in the electromotive series.
- Regular Maintenance. A corrosion protection method’s effectiveness depends on several factors, including the quality of the protective layer, environmental conditions and the quality of the materials used.
Here we are going to learn few more things about Corrosion Protection in this article. We will be talking about “Corrosion Inhibitors” which can be used directly or made part of paints and varnishes for coating which are used for “Protective Coatings”.
In chemistry, a corrosion inhibitor or anti-corrosive is a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy, that comes into contact with the fluid. The effectiveness of a corrosion inhibitor depends on fluid composition, quantity of water, and flow regime. Corrosion inhibitors are common in industry, and also found in over-the-counter products, typically in spray form in combination with a lubricant and sometimes a penetrating oil. They may be added to water to prevent leaching of lead or copper from pipes.
A common mechanism for inhibiting corrosion involves formation of a coating, often a passivation layer, which prevents access of the corrosive substance to the metal. Permanent treatments such as chrome plating are not generally considered inhibitors, however: corrosion inhibitors are additives to the fluids that surround the metal or related object.
Corrosion protection and prevention is of central importance in all sectors of industry, because corrosion damage will lead to high secondary costs. For the coatings industry there is a high demand to stop or at least to slow down these processes especially for industrial plants. Due to increased environmental awareness of the costumers and also as a result of legal regulations (e.g. VOC-regulation) water-based varnishes will be used more extensively. However, it’s a challenge for the coatings industry to produce aqueous coatings with good corrosion protection. The efficiency of antirust coatings depends on the extent of substrate preparation. The smooth metallic, dry and grease free surface is an ideal foundation for long-term protection against corrosion. These conditions are not always met in practice since in many cases residual rust remains on the surface and in the pores. At first anticorrosive effects seem to be satisfactory. However, after some time iron panels will be destroyed by corrosion bottom-up.
Corresponding additives or corrosion-retarding pre-treatment of the substrate has to be performed if using water-based corrosion protection systems, too. Each of these methods have their pros and cons:
- Pre-treatment with primer means an additional work step
- Corrosion protection pigments need a minimum layer thickness
- Many corrosion inhibitors are not stable against pH influences and humidity.
It is therefore important to use additives which can be incorporated in nearly all coating systems, can be applied on roughly de-rusted surfaces and will guarantee long-lasting protection from corrosion. An effective protection against corrosion should a) prevent the contact of corrosive active substances (mechanical barrier effect) or interfere into the electrochemical process of corrosion so that corrosion is prevented or at least strongly delayed (electrochemical influence).
This can be realised very effectively by the formation of metallo-organic complexes, the so called chelate complexes. Chelate complexes have got large importance in nature and also in chemical analysis as extremely stable compounds (haemoglobin, vitamin B12, aluminium-detection with alizarine etc.) By forming a metal chelate further oxidation of the metal substrate is prevented by stopping any further reaction of the Fe3⊕ (s. reaction scheme). This reaction is simplified, a transformation of instable Fe-(III)-oxides into stable Fe-(II)-oxides, as a component of very hardly soluble chelate complexes. Iron-(II)-compounds are known, e. g. from iron oxide-pigments, as very stable compounds. When applied as a primer on an iron substrate with remaining rust, very stable, metal organic layers are obtained, which cannot be oxidised any more. It provides strong adhesion at the metal surface, so that bounded iron ions are not available for continuing electro-chemical corrosion any more. Furthermore, the construction of this layer will effectively prevent any further attack of oxygen on the metal below, describable as a barrier layer. A chemical compound between metal surface and organic coating is formed. The result is long-lasting protection from corrosion.
The corrosion inhibitor SCHWEGO Corrit 6831 developed by Schwegmann Company exactly acts according to these principles. It is similar to KORRODUR which has been established in the market for years. Therefore, both products offer corrosion protection for iron substrates and can be used in many coating systems (repair coating systems, DIY, one-layer rust protection systems):
- Existing residual rust is passivated by transforming it into exceptional stable metal complexes.
- The wetting of the substrate is improved.
- The adhesion of the varnish layer to the substrate is optimised.
- It can be applied on rusty surfaces – only loose rust has to be removed.
SCHWEGO® Corrit 6831 is suitable for water-based coating systems with almost all current resins and is added by 2 – 5 %. Practically every coating system can be provided with corrosion-inhibiting properties without any further changes of the formulation.
The Task Function of KORRODUR:
- Deactivation of any present rust
- Promotion of substrate wetting
- Improvement of adhesion of the coating to the substrate
- Conveying existing moisture to the surface of the film
Composition
- KORRODUR is an additive for rust protection primers and one-coat paints
- KORRODUR consists of a chelate forming agent
- KORRODUR is modified with a phosphoric acid ester compound in order to improve
- KORRODUR is diluted in a mixture of butanol, butyl acetate and glycol ether
Mode of Action
- The chelate forming agent reacts with rust and converts it to an inactive iron organic complex
- Phosphoric acid compounds support its passivating properties and contribute to the formation of a protective coating
- The wetting additive component ensures good wetting of the substrate and emulsification of existing moisture which produces good wetting of existing porous rust constituents and penetrates any existing residual rust
- The polymeric ingredient ensures the optimum interaction of all ingredients and improves adhesion to the substrate
- The solvent mixture is compatible with coating systems and favours the good and stable dispersion in the system.
KORRODUR is added to about 3 – 5 % to Solvent Borne Systems.
To test the activity of KORRODUR it is necessary to use pre-rusted test panels and to carry out accelerated weathering. The salt spray test does not correlate with outdoor weathering and should not be used.
KORRODUR does not lead to yellowing or longer drying times. Delays in drying time may occur if excessive doses are used. A slight discolouration may be possible and has no influence on the effectiveness of the product. The use of KORRODUR in pastel coloured paints should be tested beforehand.