One of the most difficult processes in manufacturing pigmented systems is the process of dispersing pigments and trying to prevent from flocculation occurring. Flocculation is the recombination of dispersed pigments that are not stabilized in dispersions. In water-based systems, the problems are exacerbated due to the high surface tension of water which makes the wetting of low surface energy pigments difficult, especially for organic pigments. Inorganic pigments like titanium dioxide are relatively easy to disperse in water-based systems and dispersants with anionic properties are usually the products of choice.
A dispersant or a dispersing agent is a substance, typically a surfactant, that is added to a suspension of solid or liquid particles in a liquid (such as a colloid or emulsion) to improve the separation of the particles and to prevent their settling or clumping
Dispersants are widely used to stabilize various industrial and artisanal products, such as paints, ferrofluids, and salad dressings. The plasticizers or superplasticizers, used to improve the workability of pastes like concrete and clay, are typically dispersants. The concept also largely overlaps with that of detergent, used to bring oily contamination into water suspension, and of emulsifier, used to create homogeneous mixtures of immiscible liquids like water and oil. Natural suspensions like milk and latex contain substances that act as dispersants.
However, as mentioned organic pigments have low surface energies but also have high surface areas, and more complex dispersants are required.
One of the most important steps in the production of pigmented coatings is the homogeneous distribution of solid pigments within the liquid binder solution. If this pigment grinding step is not optimized, a wide variety of defects can occur:
- Flocculation
- Gloss Reduction
- Color Shift
- Separation
- Settling
- Formation of Bénard Cells
Note: Bénard cells are hexagonal cells formed on the coating film due to the swirling flow resulting from solvent evaporation in thin films. The cell is subject to a movement in which the flows generate hills on the centers and holes at the cell boundaries. These flows result in the decomposition of different coating components thus accumulating at various sections of the cell leading to the formation of color and surface differences. These cells become more evident as the coating dries or hardens. They may not be observed if they are spread out during hardening/stoving. However, the cell appearance is generally fixed on the hardening film and is observed as a defect.
Properties Related to the Flow Characteristics of the Coating System, Such as
- Sagging
- Levelling
- Floating on Surface
- Rubbing Fastness
- Abrasion Resistance
- Color Value of Final Product
Can also be negatively affected.
There are generally 3 steps in the process of dispersing pigments:
- Wetting
- Dispersion (grinding)
- Stabilization
Before we discuss the 3 important steps of dispersion of pigments, we need some definitions of pigment particles.
Primary particles are single (or double) crystals and are the smallest particles obtained during pigment synthesis and their simple shapes can vary between cubic, rhomboidal, etc.
Aggregates are rigid clusters of primary particles joined by crystal faces. Due to the high adhesion forces, dispersion does not cause aggregates to separate into primary particles.
Agglomerates are clusters of primary particles or aggregates that are loosely joined by crystal edges due to the action of van der Waals or Coulombic forces. These can be broken down, so the entire process of pigment dispersion is to divide agglomerates into primary particles and aggregates. In practice, large agglomerates usually end up being divided into smaller agglomerates.
1) Wetting is the first step of the dispersion process in which air and moisture are displaced from the pigment surface by the dispersing agent. This is done through preferential adsorption. The efficiency of wetting depends primarily on the surface tension properties between the pigment and the vehicle, as well as the viscosity of the resultant mix. The air must be fully removed and the pigment surrounded by the liquid medium.
The action of wetting can cause surface tension to be lowered.
This means that a liquid with low surface tension will wet the pigment surface better than a high surface tension liquid so that a dispersant that acts as a wetting agent in this first step will lower the surface tension.
2) Dispersion (grinding) stage is where the primary purpose is to break down pigment aggregates and agglomerates to their optimum particulate size by milling machines or in the case of inorganic pigments the use of a high-speed mixer using a saw tooth blade. The dispersant additive speeds up the wetting of the newly created surfaces and minimizes the pigment–pigment interaction to produce a lower viscosity dispersion. During this process, the pigment particles are stabilized against flocculation and without this stabilization, the primary particles would re-agglomerate releasing the energy that was introduced into the dispersion by the grinding process. Dispersants reduce the surface tension, and the work needed to disperse the pigment is less. This means that the grinding time is reduced.
3) Stabilization is the most critical stage of the process and the dispersant needs to have good wetting and dispersing properties to be able, over a period of time, to keep the particles separated. If this does not happen then the particles can re-form to form flocculates. Flocculation is due to the London – Van der Waal attractive forces occurring between the particles. These forces are only effective over very small distances. In this instance, Brownian molecular motion causes particles to collide many times and creates flocculation.
The dispersant which anchors onto the pigment surface helps to repel the attractive forces between the pigment particles and stops the formation of agglomerates. This requires the dispersant to have groups which interact strongly with the pigment surface by a variety of bonding mechanisms. This stabilization of the pigment ensures that complete wetting and separation of the particles has been reached and that the pigment particles are homogeneously distributed in the medium.
If the dispersion has not been stabilized, flocculation may occur as a result of clumping together of the pigment particles. Flocculation is generally a reversible process. Flocculates typically break down when shear is applied and will form again when the shear is removed. If the pigment dispersion is not stabilized by the resin component in the dispersion, then the use of surfactants or dispersants can be considered
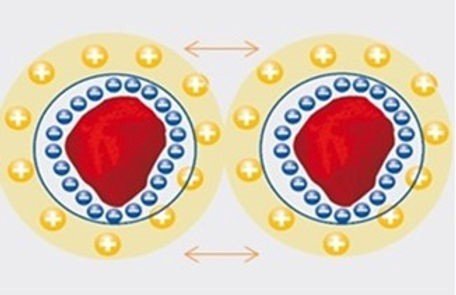
Electrostatic Stabilisation – this occurs when particles have the same electrical charge and hence cause repulsion between the particles. What generally happens is stabilisation through an electrical double layer, in which each layer has an equal charge and when two particles come together, their respective charged double layers overlap and repulsion occurs.
The Double-Layer Concept
When suspensions of electrostatically charged particles are placed into an electric field of field strength E, they will migrate to the oppositely charged electrode with the velocity n. The quotient of n/E is called the is determined by the sum of the van-der Waals attractive and the electrical double layer repulsive forces. The attractive and repulsive forces depend on the interparticle separation. If the double layer is stronger, repulsion is the main force and the dispersion is stable. However, if the double layer is affected by say the addition of salts, then the attractive forces are the main factor and the dispersion is broken up.
Traditionally, zeta potentials were determined using micro electrophoresis. The electrophoretic mobilities of the particles were measured under a microscope while an electrical field was applied. The stability of electrostatic interactions can be measured by their zeta potential (ζ) Zeta potential is the scientific term for electro-kinetic potential and is measured in volts (V) or millivolts (mV).
Electrostatic stabilization works for water-based systems but for solvent based and UV based systems steric stabilization plays a key role in preventing particle flocculation. This type of stabilization also occurs in water-based systems.
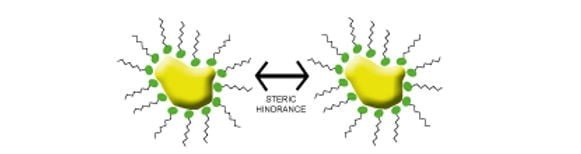
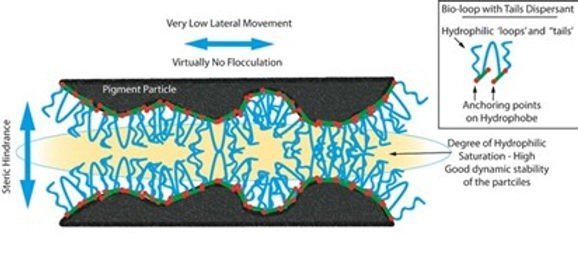
Steric Stabilization – In this type of stabilisation polymer molecules is the generally preferred choice. They have two key features; one is a pigment affine (anchor) group and a second binder compatible group. The anchor group attaches itself to the pigment surface and ensures strong adsorption of the dispersing additive. The binder compatible group reaches out into the solvent / UV monomer binder. Together these two features create a stabilization effect on the system.
As this process relates to energy the Gibbs – Helmholtz equation is useful for understanding how this process works.
In its simplest form the equation is:
G = H – T.S
G = Gibbs free energy
H = Enthalpy of the system
T = Temperature
S = Entropy of the system
Taking the temperature to be constant then you can say:
ΔG = ΔH – T. ΔS
If the pigment particles get too close to each other their mobility is reduced and the entropy is lowered. To balance the entropy loss the pigment particles, move away.
In the above equation, flocculation is prevented by having a positive value for ΔG.
To be effective as a polymer dispersant the additive must be highly solvated as possible and be highly compatible with the binder solution. If this is not the case then flocculation is possible. Steric stabilization is useful for both water based and non-water-based systems.
Electrosteric Stabilization – Is a combination of electrostatic and steric stabilization. Specifically, this type of stabilization features adsorbed polymers like in steric stabilization, however the adsorbed polymers have non-negligible electrostatic charge. Therefore, we get significant double-layer repulsion.
Limits Of Stabilization – There are instances when the stabilization is compromised. This can happen at high temperatures where the primary particles move closer to each other within the range of attractive forces and the pigment flocculates. In most cases these flocculated particles can be easily separated by the application of low shear forces. One of the most common problems in the coatings market is pigment shock or solvent shock.
In pigment shock the issue is caused by adding too quickly film formers/ binders to the newly dispersed millbases in the let-down stage. The solvent diffuses out of the paste phase – low binder content into the phase which is high in binder content, to balance the concentration. This however causes the paste phase to dry out and the pigment particles to flocculate. Pigment shock is only reversible when the polymer layer adsorbed on the surface of aggregates and primary particles in the mill base ensures sufficient stabilisation for the conglomeration to behave as a form of flocculation the risk of shock can be reduced if the concentration of the film former chosen in the material to be dispersed is as high as possible and if the concentrated film former solution is added very slowly during this phase but also stirring vigorously.
Solvent shock is a similar process in that the solvent diffuses into the pigment paste, which is now richer in binder and thus reduces the stabilizing effect of the film former molecules.
Solvent shock is the occurrence of flocculation due to inaccurate solvent application rates during the production of coatings. The solvent molecules, rapidly penetrating the mill base, strip the pigment particles of their stabilising polymer layer. Pigment re-agglomeration ensues and nullifies the dispersion effect This change is therefore largely irreversible. Carbon blacks and some finely-divided organic pigments are particularly sensitive to shock.
Types of Dispersants:
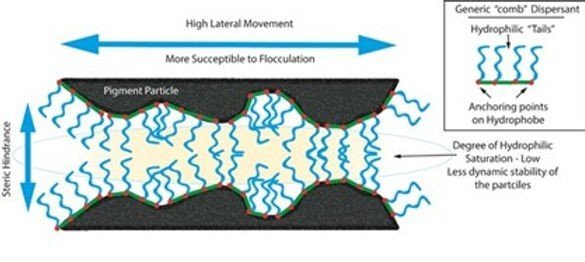
1. Low Molecular Weight Products – The original dispersing additives for pigments have been around for many years and consist of both hydrophilic and lipophilic groups. These conventional dispersants (surfactant type) tend to have molecular weights of < 2000g/mol and are characterised by their hydrophilic group, cationic, non-ionic, etc. These conventional low molecular weight polymers tend to be used for inorganic pigments and extenders / fillers. The mode of action is that the polar head of the dispersant forms hydrogen bonds with the pigments causing the particles to keep separated and stabilize the system. As these dispersants have low molecular weight, they will not give sufficient steric hindrance. These types of dispersants are good for the inorganic pigments especially the metal oxide types as they contain positive metal ions and negative oxide ions. However due to the action of these low molecular weight dispersants they are not useful for organic and black pigments.
Lankem Low Molecular Weight Dispersants
| Product Nature | Product Name |
| Anionic | Lansperse DS 80 |
| Non Ionic | Lanasperse DS 200 W |
| Amphoteric | Amphokem CADP |
These low molecular weight dispersants can lower the interfacial tension between the pigment and the binder solution. They act as surface active agents because they have two contrasting groups relating to solubility or polarity. For water-based systems the polar group is a hydrophilic group and the non-polar is a hydrophobic or lipophilic group. In non-water-based systems the polar group is called the oleophobic group and the non-polar one is the oleophilic group.
With these lower molecular weight dispersants their effectiveness is related to the absorption of the polar group onto the surface of the pigment and these anchoring groups can be amino, carboxylic, sulphonic, phosphoric acids and their salts and others.
The surfactant nature of these dispersants can cause certain defects like foaming, problems with intercoat adhesion and water sensitivity.
However, when it is necessary to stabilize organic pigments (or also fine-particle carbon black pigments) against flocculation, these additives show considerable weaknesses. A durable and permanent adsorption onto the pigment surface is of utmost importance in order for additives to be effective, as this is the only way that a stable protective envelope can be formed. Inorganic pigments are ionically constructed and display relatively high surface polarities, thus making adsorption of the additives relatively easy. Organic pigments have a completely different structure. Here, the pigment crystals are made up of individual molecules which are also predominantly non-polar and held together by means of intermolecular forces. As a result, organic pigments have very non-polar surfaces and therefore make proper adsorption of conventional additives rather difficult. On account of the negligible interactive forces between the adhesive groups and the pigment surface, the dispersing additives are able to fall away from this very easily and there is no stable protective envelope around the pigment particles. In practice, this means that in many cases organic pigments are insufficiently deflocculated and stabilized using low-molecular weight wetting and dispersing additives. Moreover, this is compounded by the fact that the fine-particle organic pigments have more of a tendency to flocculate than the coarse-particle inorganic ones. As the entire pigment surface must be covered with additive molecules and, on account of their smaller particle size, the organic pigments have a greater specific surface, considerably higher additive doses are required. High additive quantities can have a negative effect on the coating film properties (e.g. hardness, water-resistance).
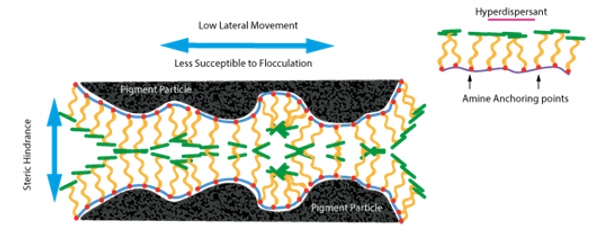
2. High Molecular Weight (Polymeric) Dispersants
These types of dispersants work by steric stabilisation due to their high molecular weight. Also, they have several types of functional groups which have an affinity for the pigment surface. Due to the greater molecular weights of these dispersants, they are attached to several sites on the pigment surface and hence form very stable adsorption layers. Optimum stabilization is achieved with excellent compatibility with the binder giving a well solvated polymer chain. If, however there is only one functional group per molecule of dispersant, then this leads to flocculation. As there are a few pigment types different functional groups are required in the dispersant for optimum stabilization.
Many inorganic pigments have high surface polarities due to their ionic nature and the dispersants are easily adsorbed onto the surface, for example titanium dioxide can be slightly basic and hence interaction with acid groups on dispersants is very strong.
Carbon black is one pigment that is different in that its surface area is many times that of organic or inorganic pigments. The surface area is highly acidic and hence nitrogen containing dispersants are some of the best types for excellent stabilization. The typical groups on the surface of carbon black are oxygen containing groups like carboxyl, quinones, phenol, ketones etc.
Carbon black is one pigment that is different in that its surface area is many times that of organic or inorganic pigments. The surface area is highly acidic and hence nitrogen containing dispersants are some of the best types for excellent stabilization. The typical groups on the surface of carbon black are oxygen containing groups like carboxyl, quinones, phenol, ketones etc.
The high-molecular weight wetting and dispersing additives have indeed been specially developed for organic pigments, but are actually just as suitable for inorganic pigments and particularly also for stabilizing pigment blends.
3. Aqueous Coating Systems – Hyperdispersants
In aqueous systems which are based on emulsion binders and primarily for emulsion paints and plasters which are used in the area of architectural coatings, the pigments are predominantly stabilized by electrostatic repulsion. Ammonium salts of polycarboxylic acids are frequently used.
In principle, aqueous systems that are based on water-soluble binder solutions or combinations of such binder solutions with binder solution emulsions (hybrid systems) can also use electrostatic repulsion for pigment stabilization. However, in practice it is found that steric stabilization with polymeric wetting and dispersing additives is frequently preferred, especially in high-quality industrial coatings. The mechanism works the same way as in solvent-borne coatings, the only requirement being that the polymeric additives must be polar enough to ensure compatibility with the aqueous surroundings. It is not necessarily desirable for such additives to be water-soluble since too high a polarity could adversely affect the durability of the coating film.
Traditional Types of Dispersants
1. Typical Comb Type Dispersing Agent – Lansperse DIS145/ Lansperse DS80/ Lansperse DS200W
2. Typical Hyperdispersant Dispersing Agent – Example: Lansperse UV51/ Lansperse SL 58/ Lansperse SL 18
The new BioLoop dispersing agents
3. Straight BioLoop – Example: Lansperse BIO868
4. Bioloop with Tails and Loops – Example: Lansperse LT87
Common Dispersants Used in Processing – Divided on the basis of Molecular Weights
|
Low Molecular Weight |
Large Molecular Weight |
| Sodium pyrophosphate | Poly(acrylic acid) (PAA) |
| Ammonium citrate | Poly(methacrylic acid) (PMAA) |
| Sodium citrate | Ammonium polyacrylate |
| Sodium tartrate | Sodium polyacrylate |
| Sodium succinate | Sodium polysulfonate |
| Glyceryl trioleate | Poly(ethylene imine) |
| Phosphate ester | Menhaden fish oil |
| Random copolymers | |
| Comb polymers |
Incorporating the Additives
Additives are frequently added to the coating during the finalization phase. However, this procedure is not suitable for wetting and dispersing additives. As these additives are needed to accelerate pigment wetting and pigment dispersion, they must be added to the mill base and ground with it. Only in this way can they be fully effective. If, in exceptional circumstances (e.g. for batch correction purposes), it is necessary to add the additives later on, they must be incorporated using as high as possible shear forces; it is preferable to run the system through the dispersing unit again. Nevertheless, you will find in the majority of cases that wetting and dispersing agents which have been added in this way are less effective and require greater doses.
Dosage
How much additive do you need to add? The correct dosage is key to the effect. Since the additive is designed to attach to the pigment surface, the required dosage of additive depends upon the amount of pigment surface present. Apart from a few exceptions, calculation formulas which link the additive dosage with, for example, the BET surface of the pigments or the oil number, are not particularly reliable and should be used only for specific pigment types. In practice, you would tend to base the dosage on the recommendations of the additive supplier and then develop a series of laboratory tests in order to optimize the dosage for your needs. You can use, for example, gloss and haze values of the coatings and the ∆E of the rub-up test as test criteria.
In the case of classic wetting and dispersing additives based on low-molecular weight polymers, a dosage of 0.5-2% for inorganic pigments and 1-5% for organic pigments are standard (additive delivery form based upon pigment weight). Typical additive dosages for polymeric wetting and dispersing additives are 1-10% (inorganic pigments) and 10-30% (organic pigments). In the case of very fine-particle pigments (e.g. some carbon blacks), greater dosages of up to 80 or 100% are required for very high-quality formulations. As these pigments can be found only in small quantities in the formulation, the additive dosage refers to the entire formulation, but is still not excessively high. A greater dosage will not have any negative effects on the coating film properties as the polymeric additives have a character that is similar to that of binder.
We should emphasize once again that all pigments must be stabilized in a coating formulation. Stabilization is also essential for allegedly “simple” pigments, such as titanium dioxide; otherwise, when mixed with other (well-stabilized) pigments there will be inevitable floating issues.